HW1
McMurry HW Ch 1: Prob 23ac, 24, 26, 42, 45 麦克默里(McMurry)作业第1章:习题23ac, 24, 26, 42, 45
Q1
PROBLEM 1-23 Give the ground-state electron configuration for each of the following elements: 问题 1-23 给出下列各元素的基态电子构型:
(a) Potassium (a) 钾
(c) Aluminum (c) 铝
Q2
PROBLEM 1-24 What are likely formulas for the following molecules? 问题 1-24 下列分子的可能分子式是什么?
(a) (a)
(b) (b)
(c) ? (c) ?
(d) (d)
Q3
PROBLEM 1-26 Draw an electron-dot structure for acetonitrile, , which contains a carbon-nitrogen triple bond. How many electrons does the nitrogen atom have in its outer shell? How many are bonding, and how many are nonbonding? 问题 1-26 绘制乙腈()的电子点结构,该分子含有一个碳-氮三键。氮原子在其外层有多少个电子?其中有多少个是成键电子,又有多少个是非成键电子?
Q4
PROBLEM 1-42 How many hydrogens are bonded to each carbon atom in the following substances, and what is the molecular formula of each? 问题 1-42 下列物质中,每个碳原子连接了多少个氢原子?每种物质的分子式是什么?
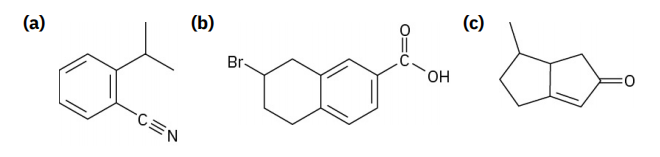
Q5
PROBLEM 1-45 Why do you suppose no one has ever been able to make cyclopentyne as a stable molecule? 问题 1-45 您认为为什么没有人能够制备出稳定的环戊炔分子?

A
Q1
Q2
a. NH₂OH b. AlCl₃ c. CF₂Cl₂ d. CH₂O
Q3

在乙腈(acetonitrile)分子中,氮原子在其最外层电子壳层中共有八个电子。其中六个用于形成碳–氮三键,另外两个构成一个非成键电子对。
Q4
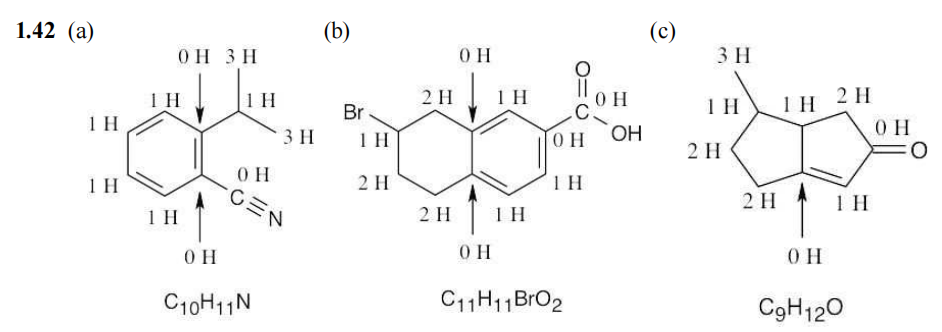
Q5
1.45 In a compound containing a carbon–carbon triple bond, atoms bonded to the sp-hybridized carbons must lie in a straight line. It is not possible to form a five-membered ring if four carbons must have a linear relationship
1.45 在含有碳–碳三键的化合物中,与sp杂化碳原子相连的原子必须处于一条直线上。如果四个碳原子必须呈线性排列,就无法形成一个五元环。